(PI: Yan Dong, Neuroscience, Pitt). Homeostatic plasticity is a physiological self-correcting mechanism through which neurons offset ‘undesirable’ alterations in excitatory signal strength. In particular, homeostatic synapse-membrane crosstalk (HSMC) has been proposed for (dys)regulating the excitability of NAc neuronal membranes implicated in cocaine craving. The goal of this R01 is to characterize the molecular substrates involved in HSMC-based dysregulation cascades. A combination of tests will be used to this end, including in vivo molecular/pharmacological manipulations, biochemistry, slice electrophysiology, and behavioral tests.
(PI: Yan Dong, Neuroscience, Pitt). Homeostatic plasticity is a physiological self-correcting mechanism through which neurons offset ‘undesirable’ alterations in excitatory signal strength. In particular, homeostatic synapse-membrane crosstalk (HSMC) has been proposed for (dys)regulating the excitability of NAc neuronal membranes implicated in cocaine craving. The goal of this R01 is to characterize the molecular substrates involved in HSMC-based dysregulation cascades. A combination of tests will be used to this end, including in vivo molecular/pharmacological manipulations, biochemistry, slice electrophysiology, and behavioral tests.
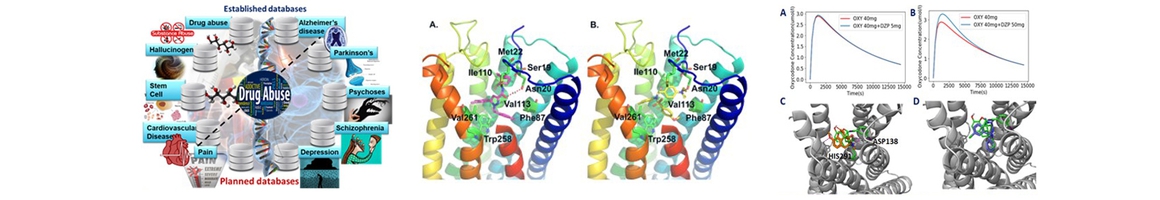
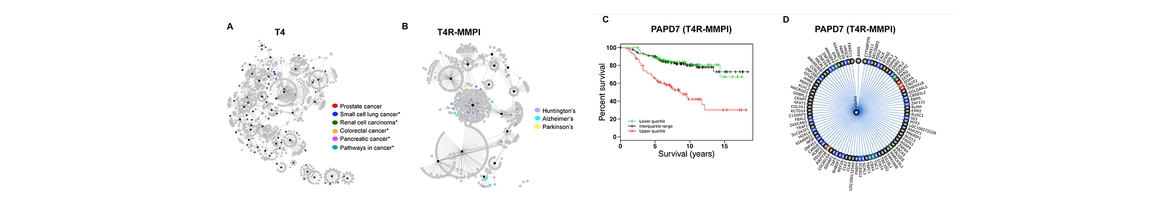
Cores A and B already initiated a collaboration with the Dong laboratory toward identifying substrates that potentially play a role in homeostatic synapse-membrane cross-talk. We adopt a multi-pronged approach to this end. On the one hand, Core A investigators utilize and further develop their developed TargetHunter and HTDocking software in the context of the constructed DA-KB database of the CDAR to map out the HSMC-based polyaddiction and dysregulation pathway. On the other, Core B will examine the druggability of potential targets, using the methodology recently developed in the Bahar lab. The Bahar lab will further search target-ligand databases to identify repurposable drugs, with the help of quantitative systems pharmacology software recently developed. Finally, Core A will apply its Fingerprint-based Artificial Neural Networks (FANN) QSAR tool to screen new small molecules targeting these proteins.
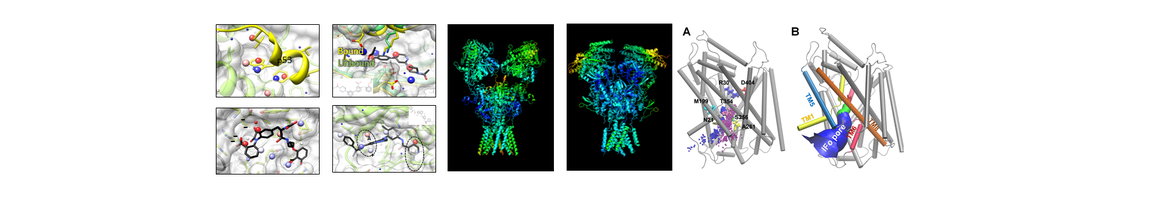
The research reported by our collaborator, Dr. Dong, indicated cannabinoid receptor 1(CB1)-expressing neurons in the NAc are critical for emotional and motivational responses. The membrane excitability of CB1-expressing fast-spiking interneurons within the NAc shell is increased after withdrawal from cocaine exposure, which may lead to increased release of GABA. Specifically, Dong’s recent study revealed that the basal functional output of the NAc is inhibited during cocaine withdrawal by multiple mechanisms associated with NAc-associated cannabinoid receptor CB1 – a system in which PI Xie/Core A has extensive experience, including CB1 ligand design and pharmacophore modeling. In collaboration with Dr. Dong, we have found that some CB ligands have polypharmacological effects, as they interact with multiple receptors including CB1, m-opioid and dopamine D1 receptors, which are known targets for DA treatment. It is highly desirable to design multi-functional small molecules to modulate these targets. Core A will employ TargetHunter and pharmacophore modeling technologies to screen and design such molecules (chemical probes). These will then be experimentally tested/validated by FRP4 for homeostatic plasticity studies with potential for DA treatments. Thus, the integrated computational predictions will provide new hypotheses that will be tested/validated by the FRP4/Dong lab. The joint effort will help us gain a mechanistic understanding of HSMC interactions, and facilitate the discovery of small molecules for restoring normal NAc core functions upon withdrawal from cocaine use.